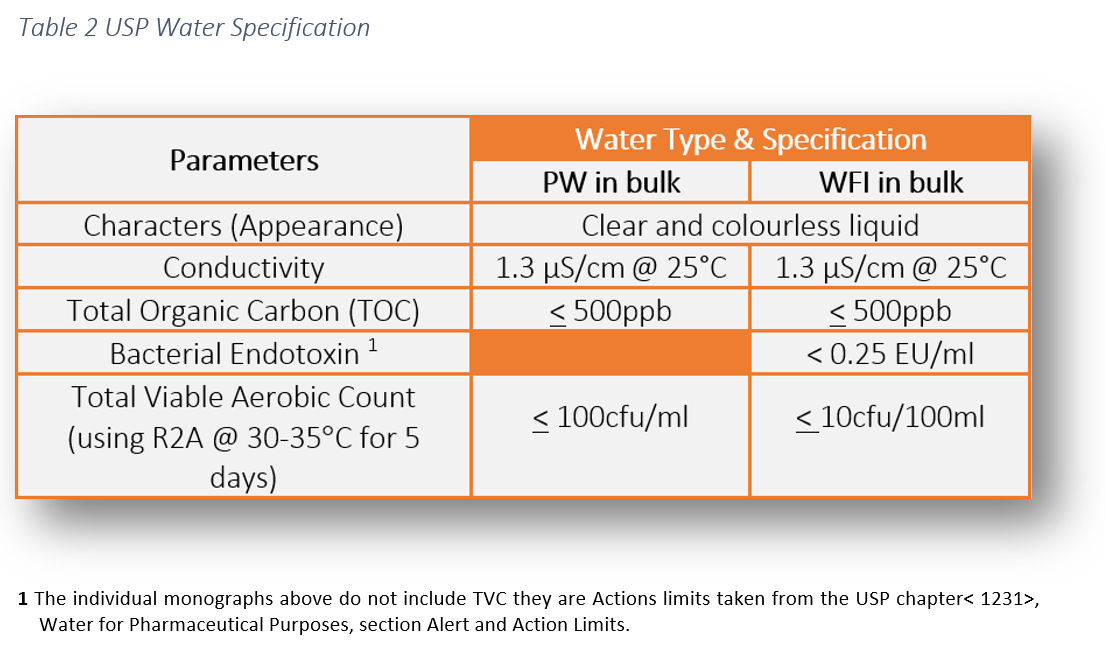
Action levels in usp 1231 100cfu ml for purified water and 10cfu 100ml for water for injection are generally considered to represent a level above which the water is unfit for use.
Water for injection usp conductivity.
We can not provide photocopies of copyrighted material.
A balancing quantity of cations such as sodium ion is.
These waters should also have fewer than 500 ppb of total organic.
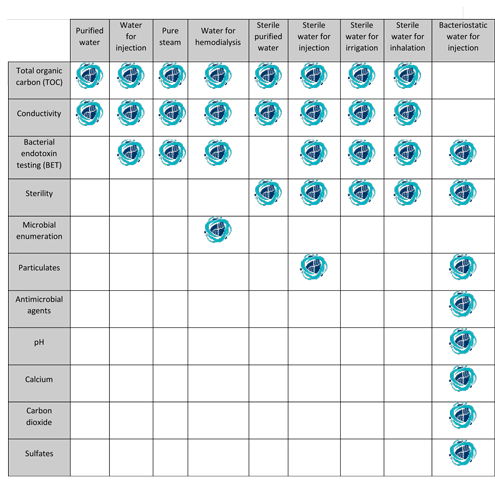
The tests for total organic carbon and water conductivity apply to water for injection produced on site for use in manufacturing.
The usp is also available at pharmacy colleges.
That is why an oos investigation must be undertaken if those action levels are exceeded.
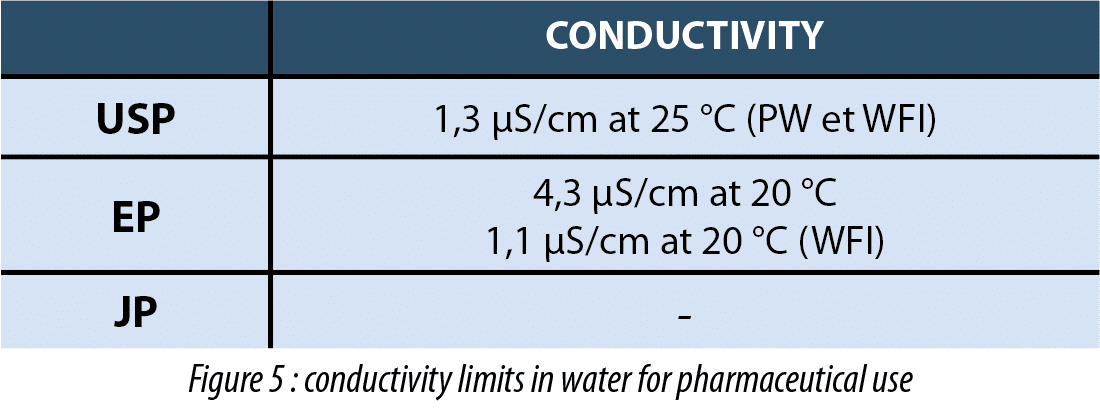
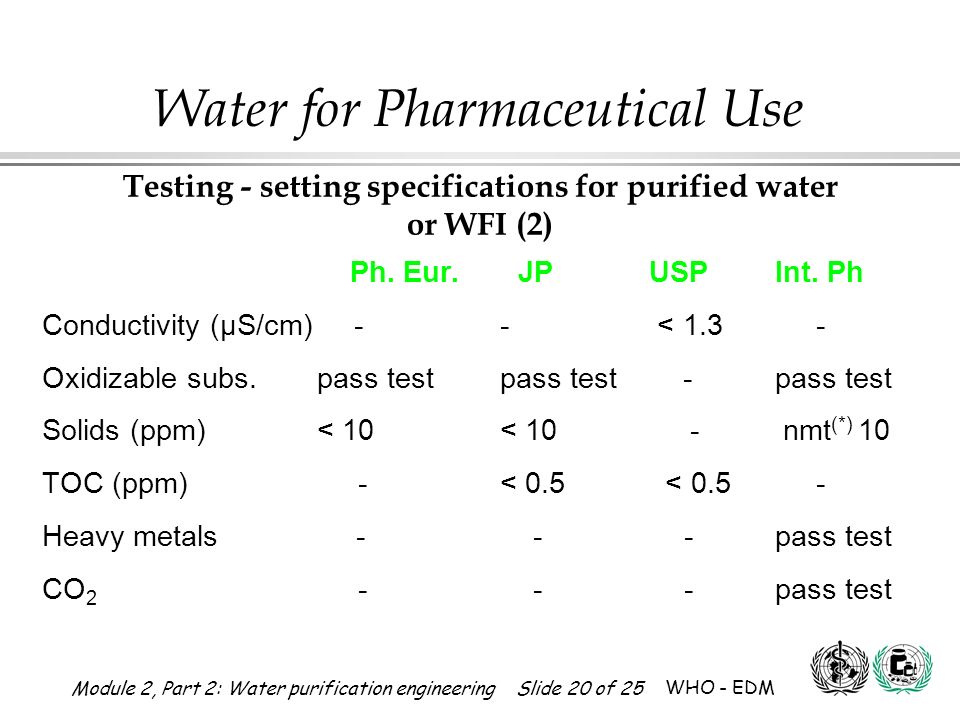
Conductivity at 25 c less than 1 3 ms cm.
Not more than 500 ppb.
Water for injection packaged in bulk for commercial use elsewhere meets the requirement of the test for bacterial endotoxins as indicated below and the requirements of all the tests under sterile purified water.
1927 1929 and 1231 water for pharmaceutical purposes p.
Clear colorless and odorless liquid.
Replacing the heavy metals attribute was considered unnecessary because a the source water specifications found in the npdwr for individual heavy metals were tighter than the approximate limit of detection of the heavy metals test for usp xxii water for injection and purified water approximately 0 1 ppm b contemporary water system.
Usp 645 this standard applies to electrical conductivity one of four critical water quality attributes defined by the united states pharmacopeial convention usp for purified water pw and water for injection wfi.
Usp bacteriostatic water for injection usp sterile water for irrigation the usp designation means that the water is the subject of an official monograph in the current us pharmacopeia with various.
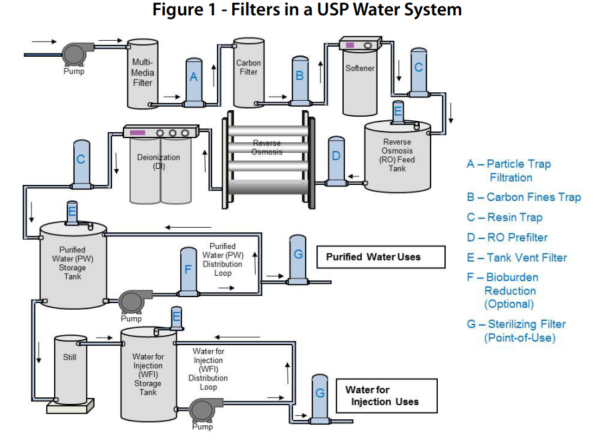
The procedure described in the section bulk water is designed for measuring the conductivity of waters such as purified water water for injection water for hemodialysis and the condensate of pure steam produced in bulk.
Some gases notably co 2 readily dissolve in water and interact to form ions which predictably affect conductivity.
Know the specification of water for injection wfi as per united states pharmacopoeia.
Water for injection by definition is water that is intended for use in the manufacture of parenteral i e.
You may purchase usp24 by calling customer service at 800 877 6733.
Know the specification of water for injection wfi as per united states.
Water conductivity is also affected by the presence of.
The usp united states pharmacopeia defines this as highly purified waters containing less than 10 cfu 100 ml of aerobic bacteria.
Usp24 contains complete versions of all pharmaceutical water monographs p.
1752 1754 and the general chapters 643 toc 645 water conductivity p.
Injectable drugs whose solvent is water.