This test method is applicable to waters that are clear in appearance and free of chemicals that will complex calcium or magnesium.
Water hardness test procedure titration method.
After 2 3 seconds the display will light up and read 0 0 so you can start using the meter.
In this article we are going to talk about the determination of hardness of water by edta titration method the causes of hardness of water is by multivalent metallic cations which react with soap to form precipitates and with certain anions present in water to form scale.
Complexometric titration is one of the best ways of measuring total water hardness.
Find the on button near the meter s display and press it down for a few seconds.
2 adapted from standard methods for the examination of water and wastewater.
The ionised form of edta is shown on the right.
Hardness total doc316 53 01317 edta titration method method 10247 100 to 200 000 mg l as caco 3 digital titrator scope and application.
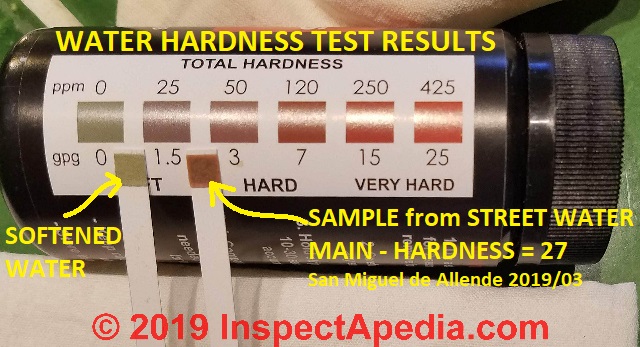
Water hardness test meters detect the number of solids dissolved in your water.
The lower detection limit of this test method is approximately 2 to 5 mg l as caco 3.
1 1 this test method covers the determination of hardness in water by titration.
1 usepa accepted when 0 020 n titrant is used.
Determining total hardness in water by complexometric titration key concepts.
In most water samples calcium and magnesium are the chief contributors to water hardness.
Water hardness can be measured using a titration with ethylenediaminetetraacetic acid edta.
The upper limit can be extended to all concentrations by sample dilution.
1 1 this test method covers the determination of hardness in water by titration.
Methods for the examination of water and wastewater standard methods 19th edition apha awwa wef 1995 with one exception as noted below.
At ph around 10 edta easily reacts with both calcium and magnesium in the same molar ratio 1 1.
Test preparation before starting as an alternative to the manver 2 hardness indicator powder pillow use 4 drops of hardness 2 indicator solution or a 0 1 g.
This test method is applicable to waters that are clear in appearance and free of chemicals that will complex calcium or magnesium.
For water wastewater and seawater.
Calcium and magnesium salts dissolved in water cause water hardness.
Edta dissolved in water forms a colourless solution.
For oil and gas field waters.
You can buy a water hardness meter online or from hardware stores.
Stability constant of calcium complex is a little bit higher so calcium reacts first magnesium later.
The lower detection limit of this test method is approximately 2 to 5 mg l as caco3.
Test preparation before starting.
Hardness of water by edta titration introduction water hardness is a measure of the amount of hard water cations in water.
The reasons for using standard methods as a reference are.
Hardness total doc316 53 01158 titration method with edta1 2 method 8226 0 25 000 mg l as caco 3 buret titration scope and application.